Nucleophilic Substitution Mechanism
Nucleophilic Substitution Mechanism: Overview
This topic covers concepts such as nucleophilic substitution reactions of alkyl halides, and unimolecular nucleophilic substitution reactions (SN1) of alkyl halides.
Important Questions on Nucleophilic Substitution Mechanism
and reactions differ from each other because of
What is the order of reactivity of following compounds towards substitutions reaction.
(i) 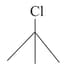
(ii) 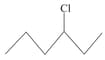
(iii) 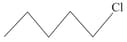
What is the order of reactivity of following compounds towards substitution reaction.
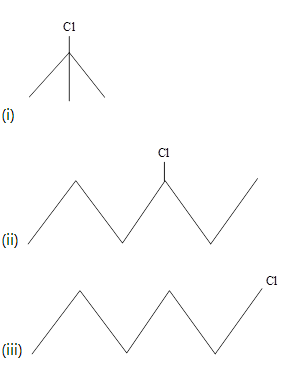
Which one of the following two substances undergoes reaction faster?
(i) 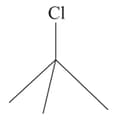 or (ii)
or (ii) 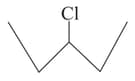
An SN2 reaction at an asymmetric carbon of a compound always gives:
A solution of (+) -2-chloro-1-phenylethane in toluene racemises slowly in the presence of small amount of , due to the formation of:
n–propyl bromide on treatment with ethanolic potassium hydroxide produces
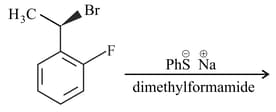
The major product of the following reaction is
Methyl propyl bromide reacts with and gives whereas on reaction with it gives The mechanism followed in these reactions and the products and respectively are:
Identify the correct order of reactivity for the following pairs towards the respective mechanism
(A) 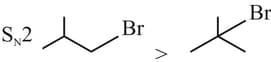
(B) 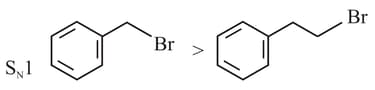
(C) Electrophilic substitution
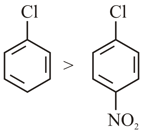
(D) Nucleophilic substitution
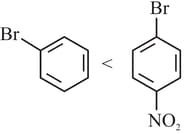
Choose the correction answer from the options given below:
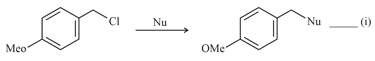
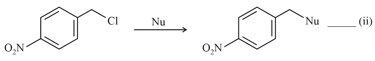
Where Nu = Nucleophile
Find out the correct statement from the options given below for the above two reactions.
Consider the reaction :
This reaction will be fastest in :
-bromooctane reacts with alcoholic NaOH to give 2-octanol as shown below.
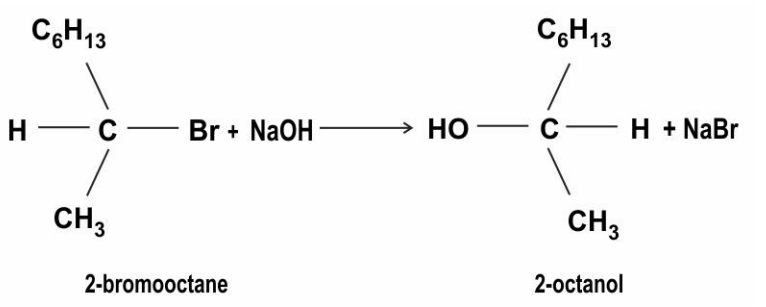
Identify the type of substitution reaction mechanism. Justify your answer?
What is the major product obtained in the following reaction ?
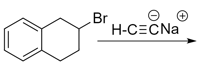
In the given reaction
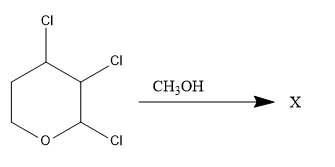
\text { Which one of the following Chlorohydrocarbon readily undergoes solvolysis?
In the following pairs of halogen compounds, which compound undergoes faster reaction?

The order of reactivity of the following compounds towards dilute aqueous in reaction is
Draw the product of each reaction
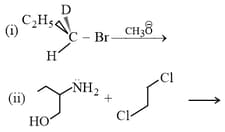
Which of the following alkyl halide can form alcohol when reacts with water?
